Viral Clearance Studies
Viral Clearance studies are a critical preventative measure to ensure the security of biological products. Accidental viral contaminations can unfortunately happen during the manufacturing process. The process must comprise manufacturing steps which reduce contamination of the final products to an acceptable level for the safety of the patient. They are required for a multitude of biotherapeutics products that include medical devices, monoclonal antibodies, gene therapy products, blood, and plasma products from donors.
Viral Clearance and the New ICH guidelines
Viral Clearance and monoclonal antibodies (mABs), plasma and proteins. (mABs)
Viral Clearance and traditional biotherapeutics, which can be classified as monoclonal antibodies, plasma and / or blood products, and recombinant proteins have dominated the majority of studies across the globe. With this extensive knowledge and history, one major addition to the ICH guidelines includes the use of ‘prior knowledge’ or ‘modular clearance.’ This concept reviews and evaluates historical data and encourages data use in place of study execution, minimizing timelines, resources, and costs. This strategy will hopefully benefit the patients by reducing the overall costs of these biologics.
“A big thank you for your reactivity and your help on this study. With these elements, we were able to make a convincing response to our regulatory authority. Thank you for your suggestions; we have integrated them into our global production strategy.
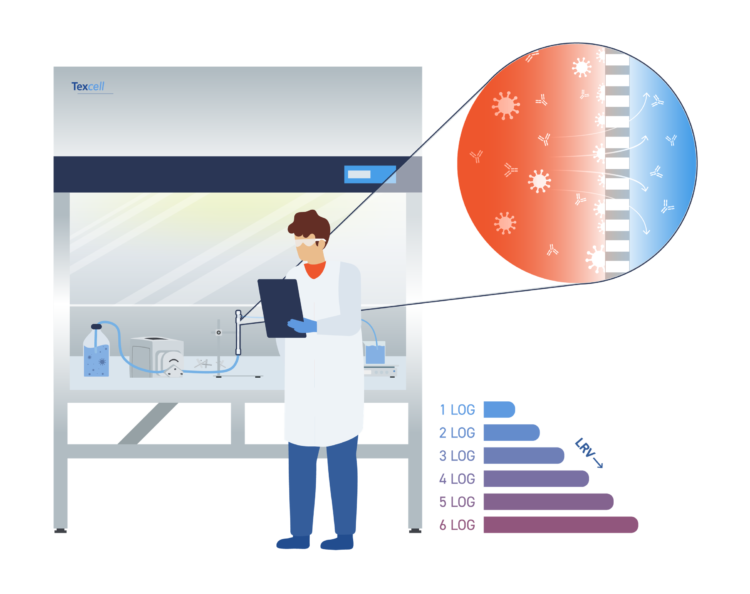
Viral Clearance and Gene Therapy Products
Gene therapy products provide a groundbreaking approach to transforming patient outcomes. The release of the ICH Q5A (r2) guidelines in June 2024 has provided enhanced clarity on the execution of viral clearance studies and the necessity of such studies for gene therapy products. In the manufacturing of these therapies, several steps are critical for viral clearance, with chromatography studies playing a central role. These studies are tailored to the specific characteristics of each gene therapy product and yield a range of log reduction values. Chromatographic processes can be further optimized through our FIO ‘Investigational’ Viral Clearance studies. These non-GLP investigative studies offer clients valuable insight into their processes, allowing them to assess the potential log reduction values achievable. Currently, 50% of our portfolio consists of gene therapy clients, including those using HEK293, HeLa, and Sf9 cell lines.
By conducting your viral clearance testing at Texcell, you evaluate that your manufacturing process effectively inactivates or removes viral contaminants, safeguarding against potential viral contamination.
Since 1990, the Texcell group laboratories, in Europe, the USA, and Asia have executed a combined total of over 10,000 Viral Clearance studies. Texcell’s experienced scientific staff can offer support, guidance, and consultation services for study design, selection of virus panel, and regulatory compliance. Texcell also provides investigational viral clearance studies “for information only (FIO)”. We tailor them to be quick, cost-conscious, and give the most critical information needed to design a robust GLP Viral Clearance testing.
Methods
Texcell can help you for:
- Maximizing effective Viral Clearance from your manufacturing platform to inactivate or remove viruses
- Performing risk assessments for Viral Clearance performance in advance of parameter setting for process validation
- Collaborating on experiment planning to get the most from FIO studies
- Designing studies for early and late phase regulatory submissions
- Preparing virus safety assessments for your regulatory submission documents
- Being an extension of your process development team in meetings with your CMC project team or your CMC regulatory team
- Designing Viral Clearance experiments for novel manufacturing processes
The Viral Clearance products experience includes, but is not limited to;
- Monoclonal antibodies
- Recombinant proteins
- Gene therapy products
- Cell therapy products
- Tissues, bone, and collagen
- Blood/plasma products
- Nanoparticles
- Vaccines
- Disinfectant Efficacy Study
- Multi-Spike Viral Clearance
Investigational viral clearance, Texcell is not limited to the manufacturing process
We define investigational Viral Clearance studies as For Information Only or “FIO”, and tailor them to be quick, cost-conscious, and give the most critical information needed to design a robust GLP Viral Clearance. Thanks to FIO viral clearance, Texcell can work with a wide range of customers such as:
- Household appliance companies (refrigerator, ironing facilities…)
- Air conditioning companies
- Car manufacturer (for air filter)
Texcell performed for these companies, studies to check a potential effect that their products could have on various viruses, including the Sars-Cov-2. Our FIO viral clearances will allow you to communicate the ability of your products to inactivate or remove any potential viral contamination.

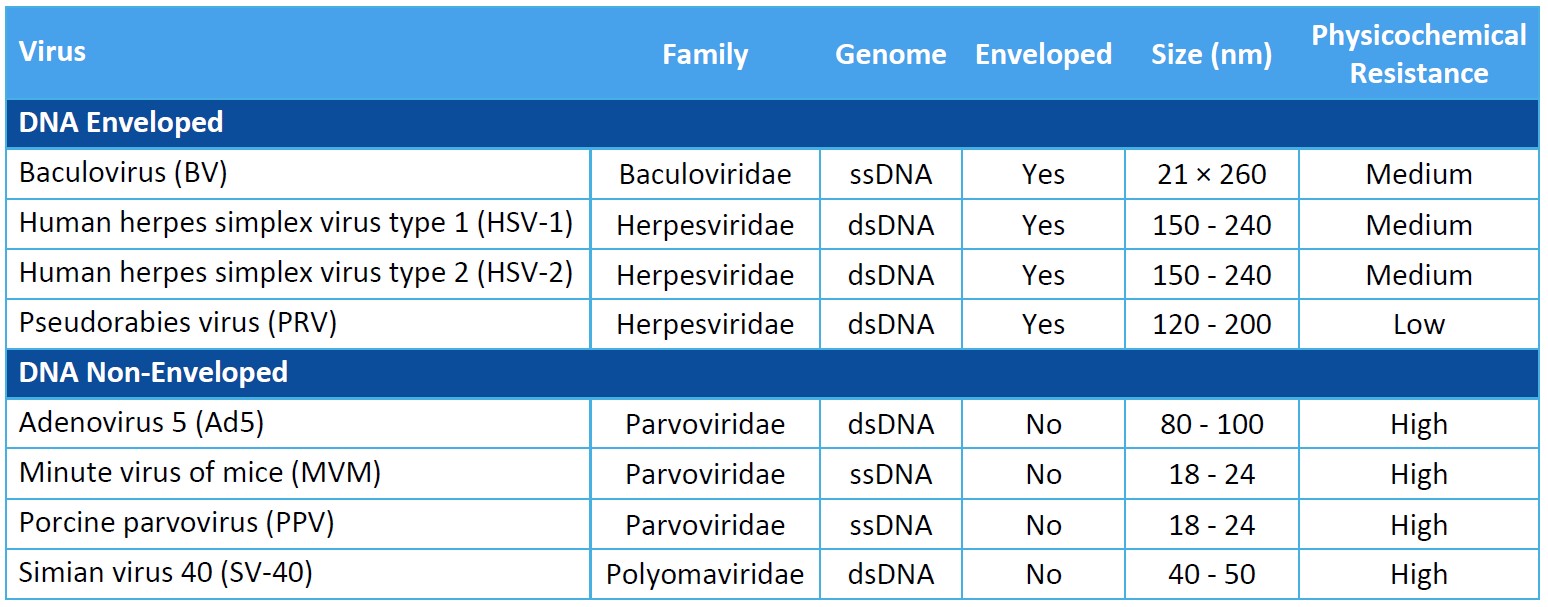
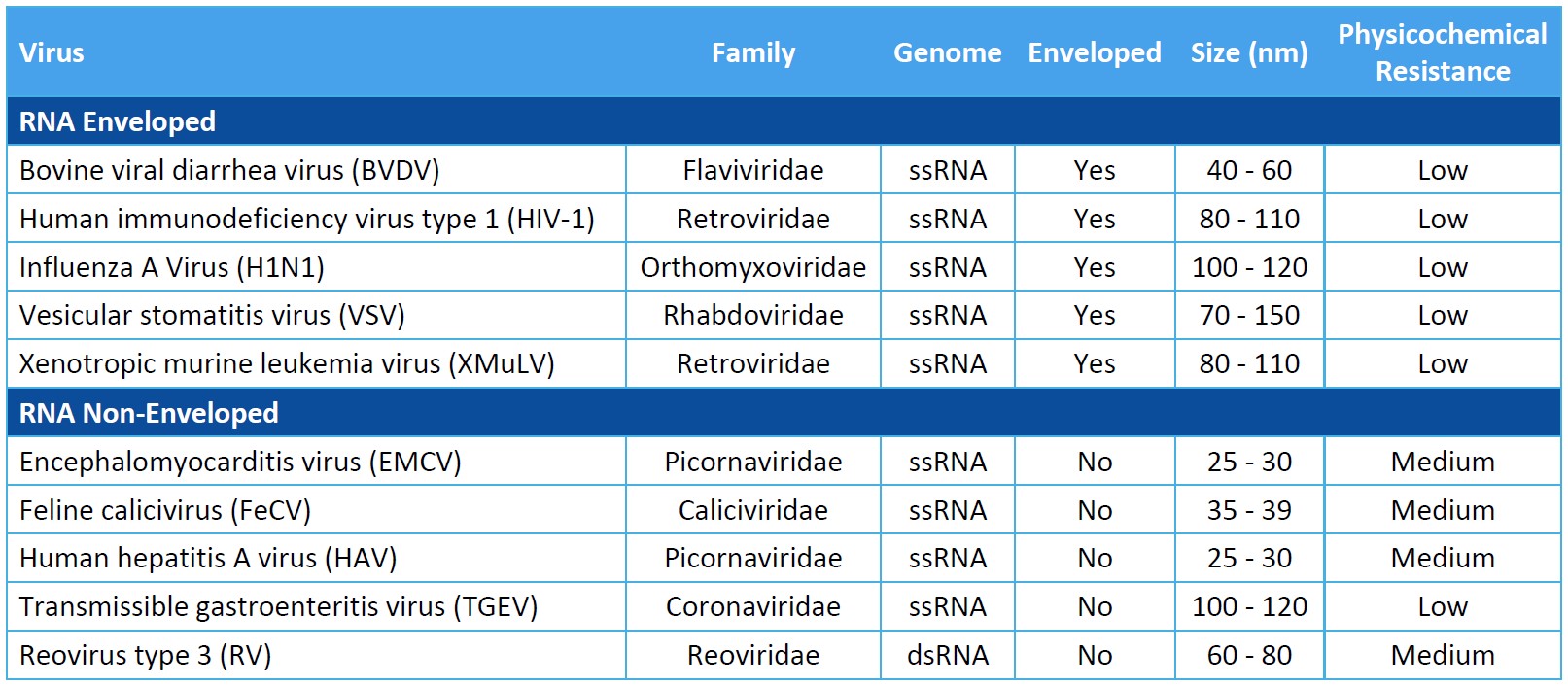
Virus Panel Selection Basics
Genome: Viral genomes consist of either DNA or RNA. DNA and RNA molecules can be double stranded or single stranded, linear or circular, and segmented or non-segmented.
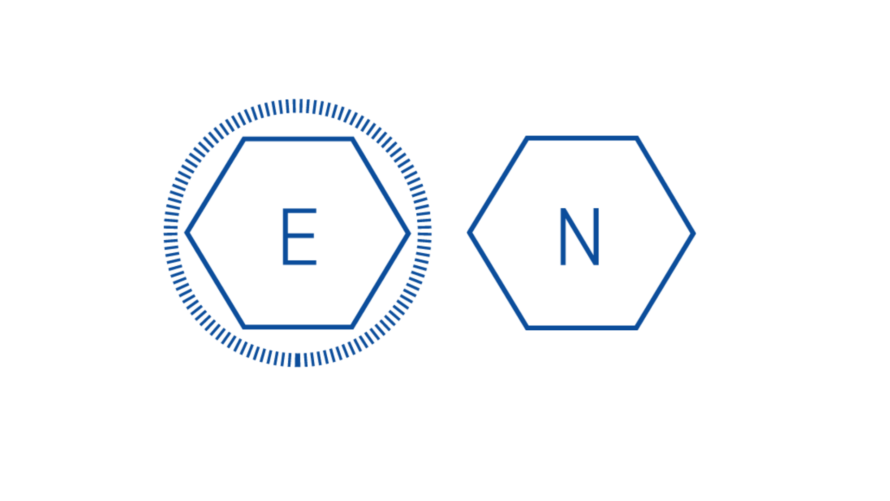
Capsid Production: The genome of non-enveloped, or “naked”, viruses is contained by the capsid itself. Enveloped viruses utilize an additional phospholipid membrane to evade the host’s immune system.
Physicochemical Resistance: A virus’s resistance to inactivation by pH, heat, and other physicochemical properties can inform a proper clearance strategy. Model viruses should represent as wide a range of physicochemical properties as possible.
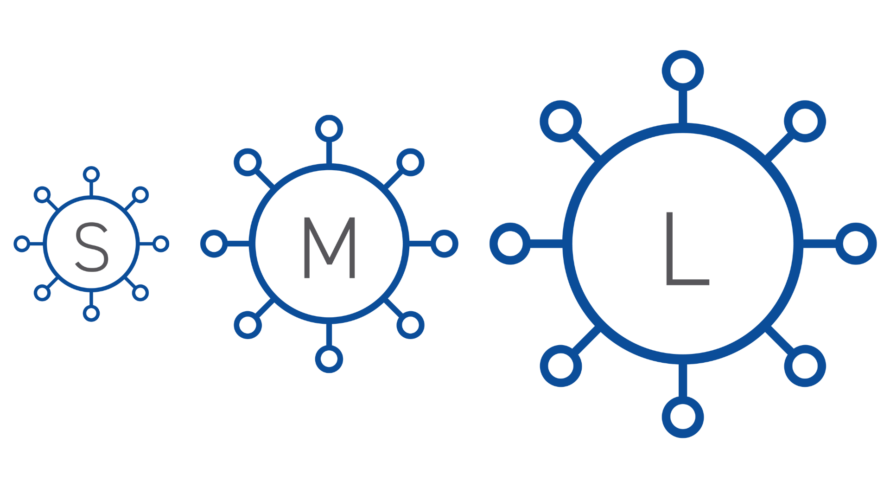
Size: Virus size will impact the design of viral clearance steps that are based on size exclusion.
For biosafety studies of therapeutics, medical devices, and disinfectants, the virus panel should include model viruses that are representative of your product’s potential virus contaminants. You should evaluate the process’s clearance of viruses known to be present in the manufacturing operation and assess the robustness of the process to clear non-specific model viruses.
When assessing the process for viral clearance robustness, you should consider including viruses that represent a range of virus characteristics and include a non-enveloped virus in the virus panel. Small, non-enveloped viruses may be more difficult to inactivate or remove and provide evidence of more rigorous process capability for viral clearance.
While selecting an appropriate virus panel for your viral clearance study, you must consider the following characteristics of the model virus.
Levels of Service
We offer three levels of service for viral clearance studies. For all levels of service, the Sponsor provides all necessary reagents (including columns and filters) to reproduce the manufacturing steps on a down scale model. Texcell team provides standard laboratory equipment (including HPLC), the selected virus panel, performs virus spiking of the starting material, and all related assays for quantitation of virus.
Level III: Premium Service
The scaled-down manufacturing process is tech transferred to Texcell. Texcell personnel execute the entire study at the Texcell laboratory.
Level II: Collaborative Service
Texcell personnel assist Sponsor in execution of the scaled-down manufacturing process at the Texcell laboratory.
Level I: Classic services
Sponsor executes the scaled-down manufacturing process at the Texcell laboratory. Texcell will oversee the virus spiking and the titrations
Prion Clearance Studies
Prions (PrPres, PrPsc, PrPtse) are non-conventional transmissible agents (NCTAs) that have become of major concern to pharmaceutical companies processing bovine or human material since the emergence of new variant Creutzfeldt-Jacob disease (vCJD).
Prion investigation studies may be required by the regulatory agencies
- Demonstrate that a cleaning procedure between each production batch can destroy prion proteins in the manufacturing equipment.
- Investigate the removal of prions during the purification of biological products (i.e., blood fractionation products, bovine extracts, etc.)
The overall level of prion reduction can be determined by deliberately adding (“spiking”) significant amounts of prion to the crude material (or to a model of the manufacturing vessels) and by demonstrating its removal or inactivation during the subsequent steps. Our highly trained staff perform these spiking experiments on scaled-down processing steps in our biosafety level 3 security laboratory.
One main strain is available for the studies, 263K (adapted to hamster), spike preparations and titration methods are also available. Please ask us to help you choose the most relevant parameters for your prion investigation study. We will advise you based on the latest technical and regulatory constraints.