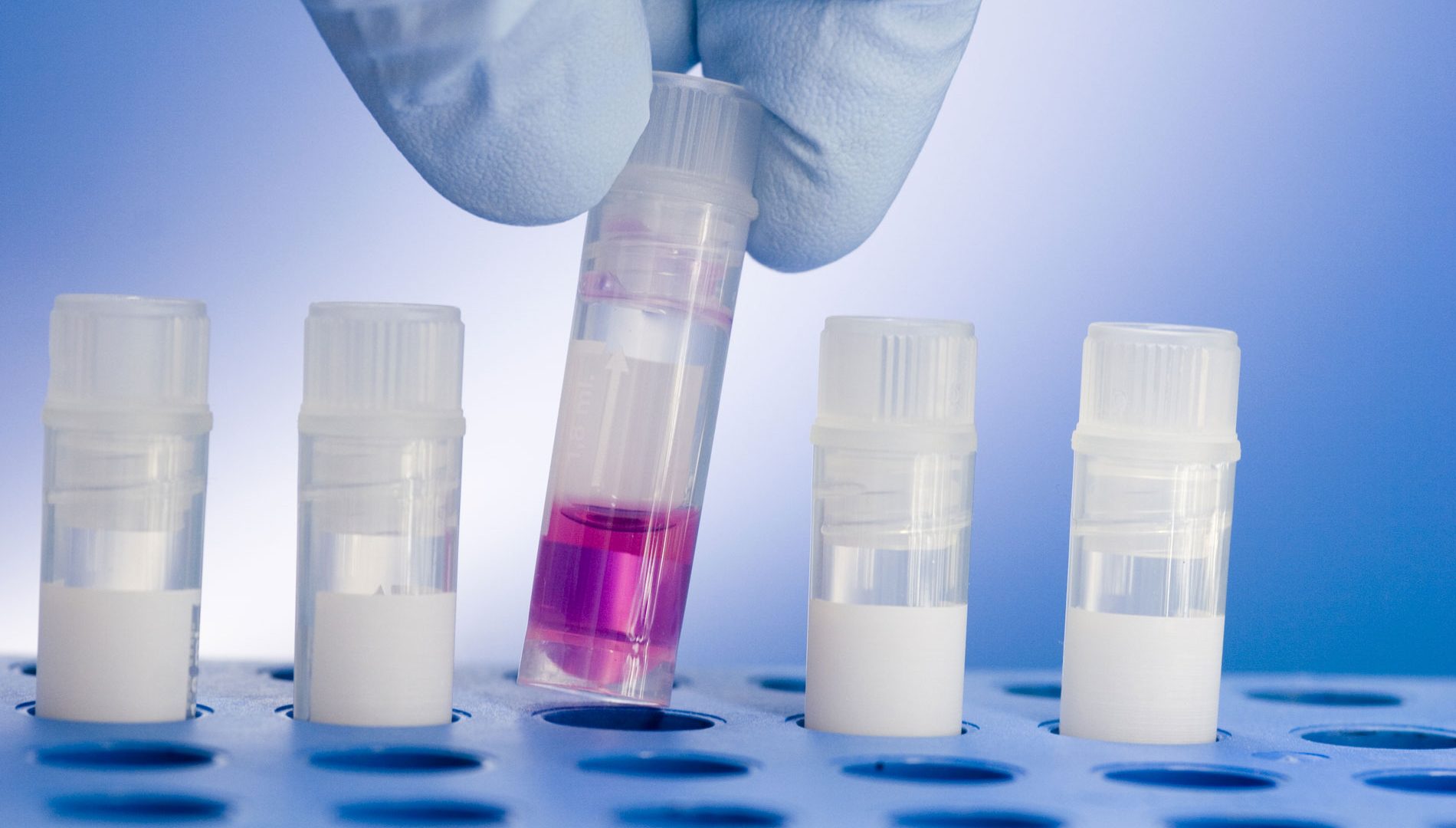
Cell Banking
Guidances on pharmaceutical development insist on the necessity to consistently deliver the intended performance of the products. Using living cells as part of the manufacturing process is a challenge. Homogeneous, large cell banks are the keys to meet these exacting requirements.
Research Cell Banking
Since 1991, Texcell has provided cell banking services to a diverse, influential client base. Our clients recognize the skill required to ensure the viability and completion of their cell lines. They have capitalized on our capabilities to produce cell lines and cell banking services. We pride ourselves in our ability to accommodate clients with cost-effective methods and rapid response to their project initiation.
Routinely performed cell culture services include:
- Generation of Recombinant Cell Lines
- Cell Line Expansion
- Cell Line Cryopreservation
- Cell Line Characterization
- Cell Banking
- Recombinant Protein Expression and Amplification
- Viral Expression and Amplification
- Custom Services
Products
A key factor in controlling production costs of biopharmaceutical proteins is the development of recombinant cell lines that produce high levels of expressed protein products.
Our team offers the following core services to expedite the development process at the R&D level:
- Transfection of recombinant plasmid DNA into mammalian cell lines via lipofectamine or PEI, or transduction of a lentiviral vector
- Stable cell selection using appropriate selective agents (G418, hygromycin, puromycin, etc.)
- Single-cell plating in 96-well plate or cloning by limiting dilution
- Cell clone validation by ELISA or qPCR
- Cryopreservation of the high-level expresser cell line clones for future use
An effective selection strategy can be designed to identify monoclonal cell lines that should stably express high levels of recombinant proteins in culture. These cells will then be ideally suited for transfer to our GMP Cell Banking prior to large-scale production of proteins in standard fed-batch fermentation processes.
Tissue and cell culture techniques are used to grow cells outside of the body in order to study cell behavior or to generate biomaterials for research or clinical use. Tissue culture is a standard technique used in biotechnology, pharmaceutical industries, academic, and government research settings.
A large number of roller bottle racks and CO2 incubators are available for adherent or suspension cell line expansion of mammalian and insect cell lines.

GMP Cell Banking
The laboratory services offered at Texcell are certified by the European Medicines Agency. GMP operations are conducted in ISO 5 and ISO 7 classified facilities and the suites can be operated under positive or negative pressure for pharmaceutical production. Our team has experience in GMP production of hundreds of cell lines including CHO, HEK, Vero, HeLa, 3T3, Lymphocytes, and Sf9.
Texcell offer a large range of laboratory services in GMP production
Texcell performs GMP cell bank manufacturing, characterization and testing. A dedicated project manager is readily available to provide you with updates on the progress of your cell bank’s GMP production, testing and characterization, as it moves from one department to the next. Texcell also offers the possibility to develop your own RCB and select your best CHO clone for pharmaceutical production.
Products
Cells used for the production of biotherapeutic products must be evaluated in accordance with the regulatory standards outlined in ICH Q5D. Texcell offers several assays to meet your cell bank characterization needs.
DNA fingerprinting by STR assay
Identity testing by cox-1 gene sequencing
Next-generation sequencing (NGS)
Other tests (like gene expression analysis, RNA 16S amplification and sequencing or karyology G-band and M-FISH) are available, dependent on the origin of the cells and the destination of the cells.
The purpose of sterility testing is to detect microbiological contaminants such as bacteria, yeast and fungi. The US and EU Pharmacopoeia, and USFDA PTC guidelines describe several techniques for the detection of bacteria in cells or cell-derived products.
In accordance with the ICH Q5D, Texcell has developed specific assays to detect bacterial and fungal contaminants for unprocessed bulks, cell banks and cells.
Available sterility assays include but are not limited to:
- Detection of bacterial and fungal contaminants utilizing a direct inoculation method
- Detection of bacteriostatic and fungistatic activity utilizing a direct inoculation method
- Detection of bacterial and fungal contaminants using a filtration method
- Mycobacteria and tuberculosis detection
Mycoplasma (Mollicutes) are one of the most common contaminants of cell lines. They can affect cell growth in culture and reduce yields of biomolecules. Texcell offers Mycoplasma testing in cell lines of human and animal origins.
Cells used to manufacture of biological products should be tested for adventitious agents including mycoplasma.
Texcell offers the detection of mycoplasma by qPCR (>90 species) according to USP, EP.
Available on demand: detection of culturable and non-culturable mycoplasma.
Banks are produced and tested in-house, significantly reducing timelines. Texcell brings quality and value to every service we perform. Reach out for a quote today and see how Texcell can best meet your needs with a cost-effective cell bank GMP production, characterization and testing. Aliquots of the cryopreserved cell suspension are filled into vials in a dedicated ISO 5 suite. Vials are frozen with a controlled-rate freeze chamber before storage.
“I would like to thank very much the Texcell’s team for its flexibility and reactivity. It has been very appreciated for our project.


